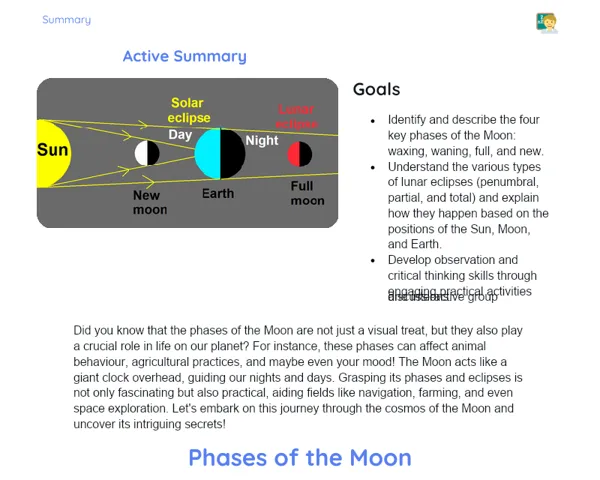
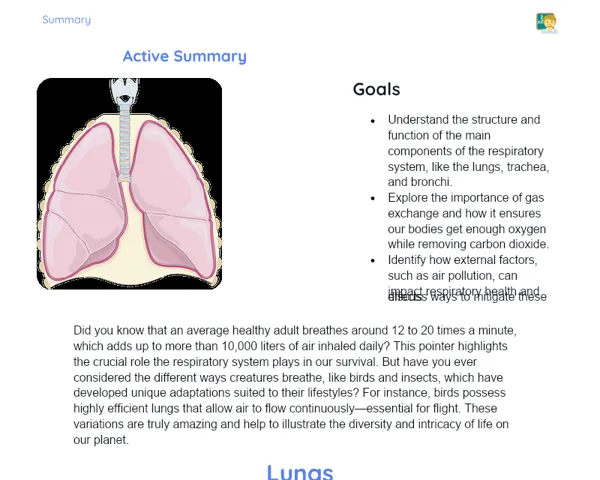
Goals
1. Understand the difference between homogeneous and heterogeneous mixtures.
2. Identify practical examples of homogeneous and heterogeneous mixtures in daily Indian life.
3. Develop hands-on skills for separating mixtures.
Contextualization
Mixtures are everywhere in our lives, from the chai we enjoy in the morning to the air we breathe. Grasping the different types of mixtures helps us better understand our surroundings and how materials come together to create new products. For instance, when we mix water and sugar, we form a homogeneous mixture where the sugar dissolves completely, creating a uniform solution. On the other hand, mixing water and sand produces a heterogeneous mixture, where the sand particles remain distinct from the water. This understanding is crucial for various professions, including chemists, pharmacists, and engineers.
Subject Relevance
To Remember!
Homogeneous Mixtures
Homogeneous mixtures, often referred to as solutions, are those where the components mix uniformly, creating a single visible phase. A common example is the mixture of water and salt, where the salt dissolves completely in water.
-
Uniformity: The components mix uniformly, resulting in a single phase.
-
Examples: Water with salt, alcohol mixed with water.
-
Importance: Crucial in the production of medicines and foods, where consistency is key.
Heterogeneous Mixtures
Heterogeneous mixtures are those in which the components do not mix uniformly, and it is possible to discern more than one phase. A typical example is the mixture of water and sand, where the sand grains are visible and separate from the water.
-
Visibility: The components are visible and can be identified separately.
-
Examples: Water mixed with sand, oil floating on water.
-
Separation: Can be easily separated by physical methods such as filtration.
Methods of Separating Mixtures
Methods for separating mixtures involve techniques used to isolate the components of a mixture. These methods can vary based on the mixture type and the physical properties of its components. Common methods include filtration, decantation, and evaporation.
-
Filtration: Used to separate solid particles from liquids in heterogeneous mixtures.
-
Decantation: A method that separates liquids of different densities or solids from liquids after settling.
-
Evaporation: Used to separate a dissolved solid from a liquid by evaporating the liquid, leaving the solid behind.
Practical Applications
-
Production of medicines: Ensuring that medicines are homogeneous mixtures for accurate dosing.
-
Water treatment: Filtration and decantation techniques are employed to purify water by removing solid impurities.
-
Cooking: Chefs leverage their understanding of mixtures to craft both homogeneous and heterogeneous recipes, such as curries and desserts.
Key Terms
-
Homogeneous Mixture: A mixture where the components are evenly distributed, forming a single phase.
-
Heterogeneous Mixture: A mixture in which the components are not evenly distributed and can be seen separately.
-
Filtration: A technique to separate solids from liquids in heterogeneous mixtures using a filter.
-
Decantation: A method for separating liquids of different densities or solids from liquids after sedimentation.
-
Evaporation: A process by which a liquid transforms into vapor, leaving a dissolved solid behind.
Questions for Reflections
-
Why is it important to differentiate between homogeneous and heterogeneous mixtures in everyday life?
-
How can the skill of separating mixtures be advantageous in various professions?
-
What challenges do we face in creating homogeneous mixtures, especially in the pharmaceutical sector?
Mix Detective
In this challenge, you'll take on the role of a mixture detective, searching for and classifying different mixtures in your home.
Instructions
-
Examine your home for three examples of homogeneous mixtures and three examples of heterogeneous mixtures.
-
Note down the characteristics of each mixture, explaining why they are homogeneous or heterogeneous.
-
Choose one heterogeneous mixture and apply a separation method (like filtration or decantation) to isolate its components.
-
Capture photos or draw the mixtures and the separation process you employed.
-
Prepare a brief report or presentation regarding your findings and share them with the class in the upcoming lesson.